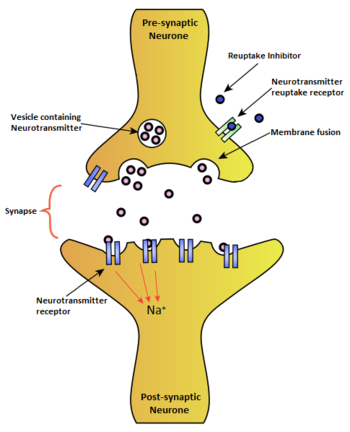
Reuptake inhibitor
A reuptake inhibitor, also known as a transporter blocker, is a drug that inhibits the reuptake of a neurotransmitter from the synapse into the presynaptic neuron, leading to an increase in the extracellular concentrations of the neurotransmitter. Various drugs utilize reuptake inhibition to exert their psychological and physiological effects, including many antidepressants and stimulants.
Reuptake is a required aspect of neurological activity which regulates the amount of neurotransmitters present in a synapse after the transmission of a neural signal. Neurotransmission occurs by transporting information across neurons by an electrical impulse called an action potential. When an action potential reaches the synapse between two neurons, the pre-synaptic neuron releases neurotransmitters to transport the chemical signal across the synapse by binding to receptors on the post-synaptic neuron. Reuptake is achieved by transporter proteins which reabsorb the extracellular neurotransmitter back into the pre-synaptic neuron for reuse. Reuptake can determine the extent, duration, and spatial domain of receptor activation.
Most known reuptake inhibitors affect the monoamine neurotransmitters serotonin, dopamine, noradrenaline, and adrenaline. However, there are also a number of pharmaceuticals and research chemicals that act as reuptake inhibitors for other neurotransmitters such as glutamate, GABA, glycine, adenosine, choline (the precursor of acetylcholine), and the endocannabinoids.
Mechanism of action
Standard reuptake inhibitors are believed to function by binding directly to the transporter protein of the neurotransmitter in question.[1] By occupying the transporter, a reuptake inhibitor competitively blocks its respective neurotransmitter from binding to the transporter protein and thus prevents it from being transported from the synapse into the presynaptic neuron. Reuptake inhibitors, like agonists and antagonists, are an example of ligand-receptor binding.[2]
Alternatively, some reuptake inhibitors bind to allosteric sites and inhibit reuptake indirectly and non-competitively. Allosteric binding may cause a conformational change in the transporter protein that prevents it from reuptaking its respective neurotransmitter.[3] Several dissociative drugs have been shown to work this way, including PCP and related drugs ketamine and dizocilpine (MK-801).[4] They appear to exert their reuptake inhibition by binding to allosteric sites on each of the respective monoamine transporters. In addition to their high affinity for the main site of the monoamine transporters, several competitive transporter substrates, such as cocaine, have lower affinity for these allosteric sites as well.[5]
Types of reuptake inhibitors
- A selective reuptake inhibitor will inhibit the reuptake of a specific neurotransmitter with negligible or no effects on the reuptake of other neurotransmitters. An example of this is the class of antidepressants known as selective serotonin reuptake inhibitors, or SSRIs. SSRIs solely target serotonin transporters (SERTs), blocking the reuptake of serotonin and increasing extracellular concentrations of the neurotransmitter.[6]
- A non-selective reuptake inhibitor will inhibit the reuptake of more than one type of neurotransmitter. An example of this is cocaine, which acts as a serotonin-noradrenaline-dopamine reuptake inhibitor, or SNDRI. Ketamine is an example of a weak SNDRI; it is non-selective because it affects the reuptake of multiple neurotransmitters.[7]
See also
External links
References
- ↑ Ravna, A. W., Sylte, I., Dahl, S. G. (October 2003). "Molecular mechanism of citalopram and cocaine interactions with neurotransmitter transporters". The Journal of Pharmacology and Experimental Therapeutics. 307 (1): 34–41. doi:10.1124/jpet.103.054593. ISSN 0022-3565.
- ↑ Dunn, M. F. (19 April 2010). "eLS". In John Wiley & Sons, Ltd. Protein–Ligand Interactions: General Description (1st ed.). Wiley. doi:10.1002/9780470015902.a0001340.pub2. ISBN 9780470016176.
- ↑ Skjaerven, L., Reuter, N., Martinez, A. (December 2011). "Dynamics, flexibility and ligand-induced conformational changes in biological macromolecules: a computational approach". Future Medicinal Chemistry. 3 (16): 2079–2100. doi:10.4155/fmc.11.159. ISSN 1756-8927.
- ↑ Akunne, H. C., Reid, A. A., Thurkauf, A., Jacobson, A. E., Costa, B. R. de, Rice, K. C., Heyes, M. P., Rothman, R. B. (August 1991). "[3H]1-[2-(2-thienyl)cyclohexyl]piperidine labels two high-affinity binding sites in human cortex: further evidence for phencyclidine binding sites associated with the biogenic amine reuptake complex". Synapse (New York, N.Y.). 8 (4): 289–300. doi:10.1002/syn.890080407. ISSN 0887-4476.
- ↑ Rothman, R. B., Silverthorn, M. L., Baumann, M. H., Goodman, C. B., Cadet, J. L., Matecka, D., Rice, K. C., Carroll, F. I., Wang, J. B., Uhl, G. R. (July 1995). "Studies of the biogenic amine transporters. VI. Characterization of a novel cocaine binding site, identified with [125I]RTI-55, in membranes prepared from whole rat brain minus caudate". The Journal of Pharmacology and Experimental Therapeutics. 274 (1): 385–395. ISSN 0022-3565.
- ↑ Sangkuhl, K., Klein, T., Altman, R. (November 2009). "Selective Serotonin Reuptake Inhibitors (SSRI) Pathway". Pharmacogenetics and genomics. 19 (11): 907–909. doi:10.1097/FPC.0b013e32833132cb. ISSN 1744-6872.
- ↑ Kohrs, R., Durieux, M. E. (November 1998). "Ketamine: teaching an old drug new tricks". Anesthesia and Analgesia. 87 (5): 1186–1193. doi:10.1097/00000539-199811000-00039. ISSN 0003-2999.