This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |

Fatal overdose may occur when benzodiazepines are combined with other depressants such as opiates, barbiturates, gabapentinoids, thienodiazepines, alcohol or other GABAergic substances.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: Norflurazepam |
| Norflurazepam | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | norflurazepam, norfludiazepam, norflutoprazepam, desalkylflurazepam | ||||||||||||||||||||||||||||||||
| Substitutive name | N-desalkyl-2-oxoquazepam | ||||||||||||||||||||||||||||||||
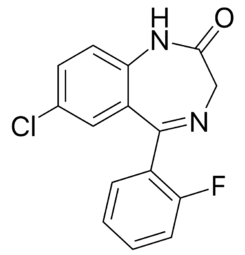
| Systematic name | 7-Chloro-5-(2-fluorophenyl)-1H-benzo[e][1,4]diazepin-2(3H)-one | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||||
| Chemical class | Benzodiazepine | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
N-Desalkylflurazepam (also known as Norflurazepam) is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs, including Flurazepam[2], Flutoprazepam[3], Fludiazepam[4], midazolam[5], Flutazolam[6], Quazepam[7] and Ethyl Loflazepate[8][9]. It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes[8]. The substance possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties.
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
It has been sold as a designer drug from 2016 onward[10], typically in pellets or particulate/powder form.
Chemistry
This chemistry section is incomplete. You can help by adding to it. |
N-Desalkylflurazepam belongs to the class of organic compounds known as 1,4-benzodiazepines. These are organic compounds containing a benzene ring fused to a 1,4-azepine. It is predictably water-soluble at a value of 0.019 g/L. The pKa1 of N-Desalkylflurazepam is ±2.5 and the pKa2 is ±11.7 [11].
Pharmacology
This pharmacology section is incomplete. You can help by adding to it. |
N-Desalkylflurazepam is a known human metabolite of Flurazepam. Its metabolism in dogs, rhesus monkeys and humans has been studied, and multiple metabolites have been identified. N-Desalkylflurazepam is further metabolized to N-1-desalkyl-3-hydroxyflurazepam in urine. [12]
It has an average half-life of about 70 hours, ranging from 47 to 100 hours[13], and accumulates during repeated dosage[14]. It may stay in the bloodstream for up to four days.[15] N-Desalkylflurazepam was observed to exhibit a high degree of plasma protein binding (> 95 %), i.e. similar to the clinically used benzodiazepines[16].
Subjective effects
| This subjective effects section is a stub. As such, it is still in progress and may contain incomplete or wrong information. You can help by expanding or correcting it. |
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects
Paradoxical effects
- Paradoxical reactions to benzodiazepines such as increased seizures (in epileptics), aggression, increased anxiety, violent behavior, loss of impulse control, irritability and suicidal behavior sometimes occur (although they are rare in the general population, with an incidence rate below 1%).[17][18] These paradoxical effects occur with greater frequency in recreational abusers, individuals with mental disorders, children, and patients on high-dosage regimes.[19][20]
Cognitive effects
-
- Analysis suppression
- Anxiety suppression
- Appetite enhancement
- Compulsive redosing
- Delusions of sobriety - This is the false belief that one is perfectly sober despite obvious evidence to the contrary such as severe cognitive impairment and an inability to fully communicate with others. It most commonly occurs at heavy dosages.
- Disinhibition
- Dream potentiation
- Emotion suppression - Although this compound primarily suppresses anxiety, it also dulls other emotions in a manner which is distinct but less intensive than that of antipsychotics.
- Memory suppression
- Thought deceleration
Experience reports
There are currently 0 experience reports which describe the effects of this substance in our experience index.
Toxicity and harm potential
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
It is strongly recommended that one use harm reduction practices when using this substance.
Benzodiazepine overdose in healthy individuals is rarely life-threatening with proper medical support; however, the toxicity of benzodiazepines increases when they are combined with other CNS depressants such as alcohol, opioids, or tricyclic antidepressants. The toxicity of benzodiazepine overdose and risk of death is also increased in the elderly and those with obstructive pulmonary disease or when used intravenously.
Long-term use of benzodiazepines has been associated with long-lasting deficits of memory, and show only partial recovery six months after stopping benzodiazepines. It is unclear whether full recovery occurs after longer periods of abstinence. Benzodiazepines can cause or worsen depression. Paradoxical excitement occasionally occurs with benzodiazepines, including a worsening of seizures.[22]
Tolerance and addiction potential
Tolerance will likely develop to the sedative-hypnotic effects (Time to full tolerance::within a couple of days of continuous use). After cessation, the tolerance returns to baseline (Time to zero tolerance::7 - 14 days). However, in certain cases this may take significantly longer in a manner which is proportional to the duration and intensity of one's long-term usage. There is clear evidence that their prolonged use will lead to dependence[23] [24] [25] [26] Sudden discontinuation of benzodiazepines is notoriously difficult; it is potentially life-threatening for individuals using regularly to discontinue use without tapering their dose over a period of weeks. It can cause seizures (which may be life-threatening in certain cases[4]) for individuals who have been heavily using them for a prolonged period of time and there is an increased risk of hypertension[27] . For this reason, it is recommended to gradually lower the daily dose over a period of time instead of stopping abruptly — a technique known as tapering.[5] For more information on tapering from benzodiazepines in a controlled manner, please see this guide.
Dangerous interactions
Although many drugs are safe on their own, they can become dangerous and even life-threatening when combined with other substances. The list below contains some common potentially dangerous combinations, but may not include all of them. Certain combinations may be safe in low doses of each but still increase the potential risk of death. Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
- Alcohol: Ethanol ingestion may potentiate the CNS effects of many benzodiazepines. The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position. Blacking out and memory loss is almost certain. If this occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- GHB/GBL: The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
- Opioids: Central nervous system and/or respiratory-depressant effects may be additively or synergistically present. The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position. Blackouts/memory loss are likely.
- Tramadol: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically present. Vomit aspiration a risk when passed out, lay down in recovery position if ingested.
- Ketamine: Both substances potentiate the ataxia and sedation caused by the other, and can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
- MXE: Both substances potentiate the ataxia and sedation caused by the other, and can lead to unexpected loss of consciousness at high doses. Place affected patients in the recovery position to prevent vomit aspiration from excess.
- DXM: Small doses of benzos can end a bad trip, but both substances potentiate the ataxia and sedation caused by the other, and this can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
- PCP: Both substances potentiate the ataxia and sedation caused by the other, and can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position. Memory blackouts are likely.
- Stimulants: Both can dull each other's effects, so if one wears off before the other, it's possible to overdose due to the lack of counteraction. If combined, one should strictly limit themselves to only dosing a certain amount of benzodiazepines per hour. This combination can also potentially result in severe dehydration if hydration is not monitored.
Legal status
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Canada: All benzodiazepines are listed in Schedule IV. [28]
Germany: N-Desalkylflurazepam is controlled under the Neue-psychoaktive-Stoffe-Gesetz (NpSG), Governing chemical groups of research chemicals, allowing to cover multiple variants. Use of covered substances is permitted only for industrial and scientific purposes. United Kingdom: N-Desalkylflurazepam is illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26th, 2016
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ Riva R, de Anna M, Albani F, Baruzzi A (March 1981). "Rapid quantitation of flurazepam and its major metabolite, N-desalkylflurazepam, in human plasma by gas-liquid chromatography with electron-capture detection". Journal of Chromatography. 222 (3): 491–5. doi:10.1016/S0378-4347(00)84153-5. PMID 7228960
- ↑ Barzaghi N, Leone L, Monteleone M, Tomasini G, Perucca E (1989). "Pharmacokinetics of flutoprazepam, a novel benzodiazepine drug, in normal subjects". European Journal of Drug Metabolism and Pharmacokinetics. 14 (4): 293–8. doi:10.1007/bf03190114. PMID 2633923. S2CID 20710732
- ↑ Descotes J, ed. (December 1996). Human Toxicology (1st ed.). Elsevier Science. p. 43.
- ↑ Vogt S, Kempf J, Buttler J, Auwärter V, Weinmann W (2013). "Desalkylflurazepam found in patients' samples after high-dose midazolam treatment". Drug Testing and Analysis. 5 (9–10): 745–7. doi:10.1002/dta.1484. PMID 23713025
- ↑ Miyaguchi H, Kuwayama K, Tsujikawa K, Kanamori T, Iwata YT, Inoue H, Kishi T (February 2006). "A method for screening for various sedative-hypnotics in serum by liquid chromatography/single quadrupole mass spectrometry". Forensic Science International. 157 (1): 57–70. doi:10.1016/j.forsciint.2005.03.011. PMID 15869852
- ↑ Nikaido AM, Ellinwood EH (1987). "Comparison of the effects of quazepam and triazolam on cognitive-neuromotor performance". Psychopharmacology. 92 (4): 459–64. doi:10.1007/bf00176478. PMID 2888152. S2CID 13162524
- ↑ 8.0 8.1 Ba BB, Iliadis A, Cano JP (1989). "Pharmacokinetic modeling of ethyl loflazepate (Victan) and its main active metabolites". Annals of Biomedical Engineering. 17 (6): 633–46. doi:10.1007/bf02367467. PMID 2574017. S2CID 31310535
- ↑ Davi H, Guyonnet J, Necciari J, Cautreels W (July 1985). "Determination of circulating ethyl loflazepate metabolites in the baboon by radio-high-performance liquid chromatography with injection of crude plasma samples: comparison with solvent extraction and thin-layer chromatography". Journal of Chromatography. 342 (1): 159–65. doi:10.1016/S0378-4347(00)84498-9. PMID 2864352
- ↑ Manchester KR, Maskell PD, Waters L (March 2018). "a and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis. 10 (8): 1258–1269. doi:10.1002/dta.2387. PMID 29582576
- ↑ Groves JA, Smyth WF. Polarographic study of flurazepam and its major metabolites. Analyst 1981; 106: 890., doi: 10.1039/an9810600890
- ↑ J. A. Groves, W. Franklin Smyth. Polarographic Study of Flurazepam and Its Major Metabolites (1981)https://doi.org/10.1039/AN9810600890
- ↑ Kulikowski, J.J., McGlone, F.F., Kranda, K., Ott, H. (1984). Are the Amplitudes of Visual Evoked Potentials Sensitive Indices of Hangover Effects After Repeated Doses of Benzodiazepines?. In: Hindmarch, I., Ott, H., Roth, T. (eds) Sleep, Benzodiazepines and Performance. Psychopharmacology Supplementum, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-69659-6_13
- ↑ Pharmacokinetic properties of benzodiazepine hypnotics. Greenblatt DJ, Abernethy DR, Divoll M, Harmatz JS, Shader RI J Clin Psychopharmacol, (2):129-132 1983 MED: 6132931
- ↑ Benzodiazepine Equivalence Table, https://www.benzo.org.uk/bzequiv.htm
- ↑ Manchester, K., Maskell, P., & Waters, L. (2018). Experimental versus theoretical log D 7.4, pK a and plasma protein binding values for benzodiazepines appearing as new psychoactive substances. Drug Testing and Analysis, 10(8), 1258-1269. https://doi.org/10.1002/dta.2387
- ↑ Saïas, T., Gallarda, T. (September 2008). "[Paradoxical aggressive reactions to benzodiazepine use: a review]". L’Encephale. 34 (4): 330–336. doi:10.1016/j.encep.2007.05.005. ISSN 0013-7006.
- ↑ Paton, C. (December 2002). "Benzodiazepines and disinhibition: a review". Psychiatric Bulletin. 26 (12): 460–462. doi:10.1192/pb.26.12.460. ISSN 0955-6036.
- ↑ Bond, A. J. (1 January 1998). "Drug- Induced Behavioural Disinhibition". CNS Drugs. 9 (1): 41–57. doi:10.2165/00023210-199809010-00005. ISSN 1179-1934.
- ↑ Drummer, O. H. (February 2002). "Benzodiazepines - Effects on Human Performance and Behavior". Forensic Science Review. 14 (1–2): 1–14. ISSN 1042-7201.
- ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. 2004 Sep;24(9):1177-85. doi: 10.1592/phco.24.13.1177.38089. PMID: 15460178.
- ↑ Owen RT, Tyrer P. Benzodiazepine dependence: a review of the evidence. Drugs 25: 385-398, 1983
- ↑ Petursson H, Lader MH. Withdrawal from long term benzodiazepine treatment. British Medical Journal 283: 643-645, 1981
- ↑ Pevnick JS, Jasinski DR, Haertzen CA. Abrupt withdrawal from therapeutically administered diazepam. Archives of General Psychiatry 35: 995-998, 1978
- ↑ Winokur A, Rickets K, Greenblatt OJ, Snyder PI, Schatz NJ. Withdrawal reaction from long term low dosage administration of diazepam: a double blind placebo controlled case study. Archives of General Psychiatry 37: 101-105, 1980
- ↑ Lann, M. A., Molina, D. K. (June 2009). "A fatal case of benzodiazepine withdrawal". The American Journal of Forensic Medicine and Pathology. 30 (2): 177–179. doi:10.1097/PAF.0b013e3181875aa0. ISSN 1533-404X.
- ↑ Branch, L. S. (2022), Consolidated federal laws of Canada, Controlled Drugs and Substances Act